Research
Introduction of research areas in Ito Lab;
- Research area 1: Hydrogels for medical uses
- Research area 2: Development of microparticles and microcapsules for medical applications
- Research area 3: Development of sheet materials and separation membranes for medical applications
- Research area 4: Application to disease treatments
Research area1:Hydrogels for medical uses
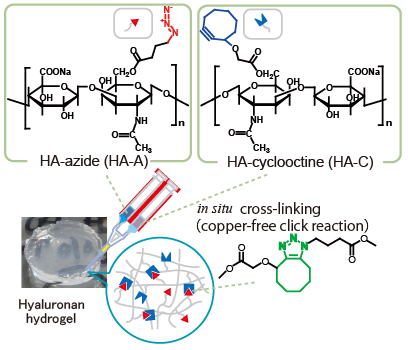
Injectable hydrogels are highly anticipated for use in minimally invasive treatments. Using natural polysaccharides and proteins as backbone, we fabricated in situ hydrogels cross-linked via biocompatible chemical reactions. Since hydrogels are easy to handle, they are anticipated for use in various areas, such as scaffolds for tissue regeneration, drug carriers, adhesion barriers, hemostatic agents and many more.
Hyaluronic acid is commonly used as a polymer backbone due to its high biocompatibility, and is important in cosmetic products, contact lens and many other health care products. Dextran, cellulose and starch derivatives, ionically cross-linkable alginate, positively charged chitosan, cell adhesive collagen and gelatin, keratin are other examples of polymer backbones.
Since natural polymers are limited in design flexibility, synthetic polymers are important to biomaterials as well. With progress in techniques for polymer synthesis, dendritic polymers have become popular in industry. With merits like low viscosity and easy surface modifications, they are gaining popularity as foam and paper coating materials. In our lab, we synthesized a novel nano-sized star polymer using chemically modified polymers as building units for future application to medically used hydrogels, as well as to colloidal dispersion system.

Other than fabrication, we also research on application process of biomaterials. The structure and functions of biomaterials are heavily dependent on both the starting material and process. As such, chemical engineering plays an important role. We are examining the gelation process using static mixers and atomizers as methods of application.

Research area 2:Development of microparticles and microcapsules for medical applications
2-1.Development of medical microparticles
There are various methods for making microparticles. Our laboratory uses the SPG membrane emulsification method. Membrane emulsification is a technique to create very homogeneous emulsions by extruding a dispersed phase into a continuous phase through a porous glass membrane with uniform pores tuned in the range of 0.5 to 100 µm. This laboratory is developing artificial oxygen carriers (artificial red blood cells) encapsulating hemoglobin and drug-releasing embolic beads.
2-2.Cell-encapsulated microcapsule
Cell-encapsulated microcapsules are expected to be one of the scaffold materials for regenerative medicine and tissue engineering. In particular, there is a long history of research to realize bioartificial pancreatic islet applications. We are developing cell-encapsulated microcapsules with diameters ranging from 100µm to 1mm, mainly utilizing alginate derivatives, using atomizers with double circular tube nozzles and the electrospray method.

Research area 3:Development of sheet materials and separation membranes for medical applications
3-1.Development of sheet materials for medical applications
Sheet and film materials can be applied on the surface of skin and tissues in the body. We develop patterned or porous sheets by utilizing molding, freeze-drying, and phase separation and evaluate their therapeutic effects. These sheet materials quickly absorb body fluids and become hydrogels.

3-2.Development of separation membranes for medical applications
Control of solutes transmission process in materials is important to develop novel drug delivery system and solutes separation system for clinical application. Artificial organs such as artificial lung and kidney are the modulated systems of hollow fiber dialysis membranes and microfiltration membranes. In addition, separation membranes are applied for downstream process of developing effective medicines as well as chromatography systems. Therefore, we develop novel separation membranes for medical applications.
Research area 4:Application to disease treatments
We are collaborating with the University Hospital and off-campus medical institutions to utilize hydrogels and micro-particles in treatments of diseases.
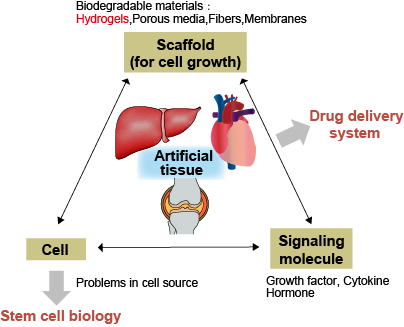
4-1.Regenerative medicine and tissue engineering
The concept of tissue engineering was proposed by a group of researchers at MIT/MGH in the 1990s. By controlling the interaction of cells, scaffolds and humoral factors, generation of artificial tissues was thought to be possible. Injectable hydrogels and microcapsules are examples of scaffold materials. In our lab, with a focus on bone and islet regeneration, we look at differentiation of cell encapsulated in hydrogels, functional expression through transplants, and angiogenesis.
4-2.Drug delivery system
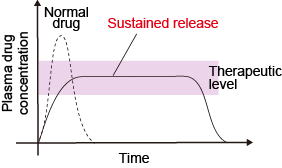
DDS refers to the technique of delivering the minimum required amount of drugs to a targeted location, at a targeting timing. One fundamental concept of DDS is sustained drug release. Drug concentrations above the limit will increase side-effects, whereas drug concentrations within the limit will not result. By maintaining the desired drug concentration range over long periods of time, not only will side effects and dosage be minimized, QOL improvements can be expected. We are researching on DDS for localized administration to peritoneal dissemination of gastric cancer, mesothelioma, and liver cirrhosis.
4-3.Tissue adhesion barriers and hemostatic materials
Adhesions of peritoneal organs to neighboring organs, or peritoneal membrane after abdominal or laparoscopic surgeries, is commonly known as peritoneal tissue adhesion. The repeated resection of the liver in hepatectomies give rise to high occurrence of adhesion formation. This greatly reduces the safety of surgeries, as well as lengthens surgery time. Taking advantage of the merits of injectable hydrogels, we seek to fabricate novel adhesion barriers possible for laparoscopic use.
Hemostasis is critical during surgical procedures. Since most available hemostats are composed of fibrin sealants and collagen-based materials, we are researching on fabrication of effective hemostats using materials not derived from the blood.
These medical devices are key to minimally invasive treatments, and indispensable to safe clinical practices.